Cell Death Mechanisms
The subversion of cellular resources and structures for virus replication and production of new virus progeny can impair the viability and integrity of the infected cell. These alterations are summarized by the term cytopathogenic effect. This differs in its extent among virus species and among the cell lines used for their study. In the case of rubella virus cytopathogenic alterations are only noted on some cell lines such as Vero. They are present through cell rounding and detachment of infected cells from the culture substrate. This results in the formation of a population of freely floating cells in the culture supernatant. Among rubella virus strains the extent of cytopathogenic alterations differs. Thus they can be grouped in low- and high-cytopathogenicity strains (Zobel & Lorenz et al., Viruses, 2018), (Bilz & Jahn et al., Journal of Virology, 2018), (Kräter & Sapudom et al., Cells, 2018). The impairment of various cellular functions after infection with rubella virus results in the induction of cell death mechanisms, which are mainly apoptotic (Claus & Mansen et al., Viruses, 2015 ). The mechanisms contributing to rubella virus-associated cell death are as follows.
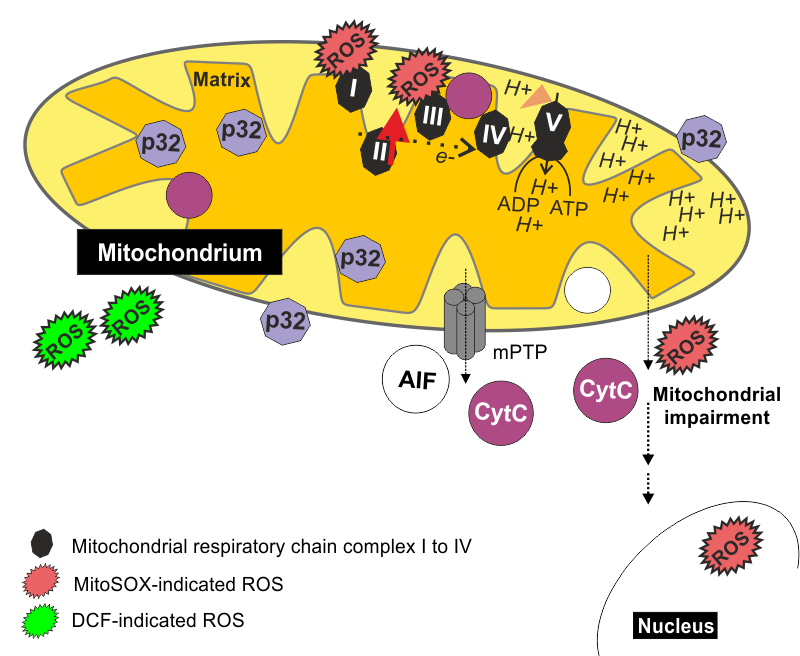
(1) Mitochondrial integrity and function are impaired during rubella virus infection (Claus & Mansen et al., Viruses, 2015). Here, the induction of apoptosis coincides with the opening of the mitochondrial transition pore, which is localized on the inner and outer mitochondrial membrane. Its opening results in release of cytochrome c, which is involved in the flow of electrons along the mitochondrial electron transport chain. The application of pifithrin µ as an inhibitor of mitochondrial localization of p53 reduced RV-associated cytopathogenic effect development.
(2) The second mechanism involves impairment of the actin cytoskeleton, which directs cellular mobility and is an important determinant of the mechanical state of a cell. The consequence of the alterations of the actin cytoskeleton by rubella virus on cellular functions was analyzed in close collaboration with Martin Kräter from the laboratory of Jochen Guck and Jiranuwat Sapudom from the laboratory of Tilo Pompe (Kräter & Sapudom et al., Cells, 2018). First we have addressed the effect of rubella virus infection on cellular mechanics through real-time deformability cytometry (RT-DC). This high throughput technology to assess cell mechanical properties was developed by the group of Jochen Guck. More information on RT-DC can be found at www.gucklab.com. In our study we have noted an increase in cortical filamentous-actin (F-actin), which was associated with a higher cellular stiffness. Only for some strains an additional reduction of stress fibers was detected, which lowered cell stiffness.
Second, the severe alterations of the actin cytoskeleton as a determinant of the stability of a cell reduced its movement capacity. This is highlighted by Figure 1 and 2, which show time-lapse movies of Vero cells in culture. Figure 1 and 2 illustrate mock (uninfected) and rubella virus-infected Vero cells after three days of infection, respectively.
(3) The third mechanism involves generation of oxidative stress, especially during infection with the high-cytopathogenicity rubella virus strains (Zobel & Lorenz et al., Viruses, 2018) Among the types of reactive oxygen species cytoplasmic hydrogen peroxide and mitochondrial superoxide were detected. MitoSOX as an indicator dye for mitochondrial superoxide was not only confined to mitochondria, but also to the nucleus, which is indicative for mitochondrial dysfunction (Zobel & Lorenz et al., Viruses, 2018). This could be due to opening of the mitochondrial transition pore during rubella virus infection (Claus & Mansen et al., Viruses, 2015 ). Accordingly, the application of MitoTEMPO and N-acetyl-l-cysteine (NAC) as scavengers for mitochondrial and cytoplasmic ROS, respectively, reduced rubella virus cytopathogenicity.
Figure 1: mock (uninfected)
Figure 2: rubella virus-infected
Bilz NC, Jahn K, Lorenz M, Lüdtke A, Hübschen JM, Geyer H, Mankertz A, Hübner D, Liebert UG, Claus C. Rubella Viruses Shift Cellular Bioenergetics to a More Oxidative and Glycolytic Phenotype with a Strain-Specific Requirement for Glutamine. J Virol. 2018; 92(17). pii: e00934-18. doi: 10.1128/JVI.00934-18.
Claus C, Manssen L, Hübner D, Roßmark S, Bothe V, Petzold A, Große C, Reins M, Mankertz A, Frey TK, Liebert UG. Activation of the Mitochondrial Apoptotic Signaling Platform during Rubella Virus Infection. Viruses. 2015; 7(12):6108-26. doi: 10.3390/v7122928.
Kräter M, Sapudom J, Bilz NC, Pompe T, Guck J, Claus C. Alterations in Cell Mechanics by Actin Cytoskeletal Changes Correlate with Strain-Specific Rubella Virus Phenotypes for Cell Migration and Induction of Apoptosis. Cells. 2018; 7(9). pii: E136. doi: 10.3390/cells7090136.
Zobel S, Lorenz M, Frascaroli G, Böhnke J, Bilz NC, Stanifer ML, Boulant S, Bergs S, Liebert UG, Claus C. Rubella Virus Strain-Associated Differences in the Induction of Oxidative Stress Are Independent of Their Interferon Activation. Viruses. 2018; 10(10). pii: E540. doi: 10.3390/v10100540.
